Good Behavioural Practices- GBP Audit
It is well established in our experience of consulting with Pharma organizations that cGMP cannot be implemented without first establishing GBP. Establishing GBP requires extensive cultural transformations. Where exactly in cultural and behavioural dimensions an organization stands will determine whether cGMP will be successfully implemented.
GBP Audit is a proprietary tool designed by UTSAV to enable Pharma organizations understand their readiness and maturity in implementing cGMP on enduring basis.
Objectives :

It’s objective is to scientifically and methodically assess variety of cultural and behavioural dimensions at all levels of management that enable and disable implementation of cGMP on lasting basis and not merely to clear statutory/ regulatory audits.
GBP-Audit report is exhaustive document that lists variety of behavioural/ cultural issues in order of criticality and importance (Critical, Major, Minor etc). Once this audit report is available, one can go about resolving the identified issues in a scientific manner.
GBP Audit also rates the organization through its GBP Score Card in terms of its maturity in GBP implementation.
Methodology and Dimensions:
Assumptions:
1. Management appreciates the value and critical importance of human element (behaviour and culture) in implementing cGMP and is serious about tackling behavioural issues.
Basic Steps:
1. We will do our primary interviewing with target group (about 10% of the entire population), selected at random as well as design, from all the levels to understand the basics of work culture and variety of important behavioural issues in the organization;
2. For instance following dimensions may be examined in minute details:
b. Current skill base of the employees in relation to the various roles and role requirements;
HRD subsystems available today to build its competency base for the present, immediate future and long term goals;
c. Leadership;
d. Learning culture and systems including knowledge management.
e. Performance management system;
f. Work Culture of the organization including ownership and responsibility;
g. Work Climate and employee motivation levels;
h. Values as practiced at middle and lower rungs of employees;
GBP Score Card
This is our proprietary Score Card to give clear picture of Behavioural and cultural based scoring of above dimensions as they relate to implementation of cGMP. This Score Card will grade the organization from Level I to Level V maturity. Level V maturity is the highest level of excellence in GBP that an organization can aspire for. Level V maturity is a clear indication that cGMP will be enduringly implemented in its true letter and spirit by the organization.
Level I: Organization Does not know that it does not know about GBP
Level II: Organization knows that it does not know about GBP and is also not implementing GBP
Level III: Organization knows and is working towards implementing the basics about GBP
Level IV: Organization values and implements basics and advance details about GBP
Level V: GBP is in the DNA of the organization and its every employee.
Most important, the grading from GBP maturity Level I to maturity Level V of the organization will spur the organizations to excel to achieve the highest Level of maturity.
Audit Report for Top Management:
1. Our audit report is an exhaustive document that clearly lights the path towards GBP implementation in the organization. It will have clear actionable points which can be implemented by the top management with or without external help. Most important the grading from maturity Level I to maturity Level V of the organization will spur the organizations to excel to achieve the highest Level of maturity.
2. It also shows the roadmap and timeline to implement the steps. Of course it will be purely on behavioural and cultural dimensions.
3. It will be a confidential document only for the top Management.
Expected Time:
It takes usually three to five days to conduct the audit and another three days to make the report. Report will be made available within two weeks of the GBP audit and further presentation on the same can be made on request and additional costs.
Commercials:It depends on the size of the plant and number of plants. Kindly ask for custom quote for your organization.
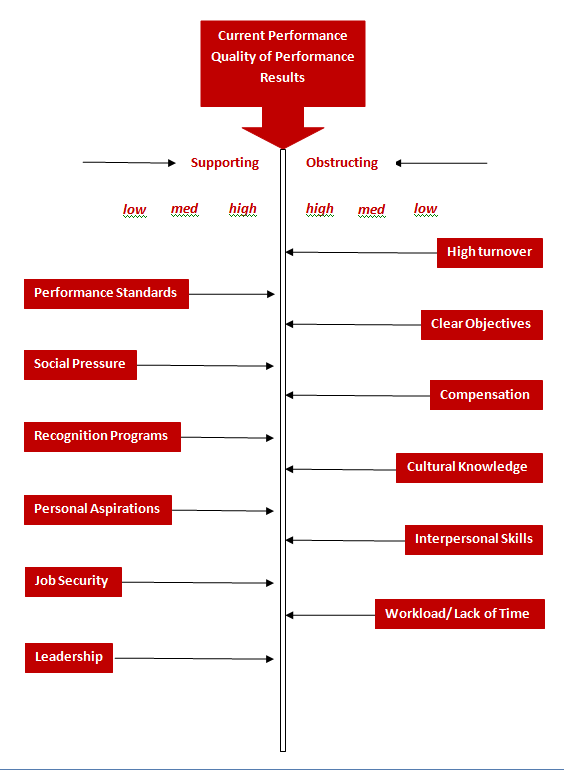
The GBP Audit Process: Sample of issues on Performance
UTSAV Expertise
• Well researched GBP Scorecard
• Use of specialized instruments and methods
• Providing detailed action plan to improve performance through GBP
• Developing internal assessor capabilities
• Counseling and facilitating development action plans • Psychometric tests (developed in house)
Fundamental Values to Achieve GBP- Win-Win of Conflicting Dimensions

Source: Above table is from Built to Last by Jim Collins

Good Behavioural Practices
“At the heart of enduring success for any Pharmaceutical company lie Good Behavioural Practices- GBP”
Good Behavioural Practices - GBP emerged from our Behavioural Consulting with Pharmaceutical manufacturing Organizations. There is a business compulsion of implementing cGMP (Current Good Manufacturing Practices) in these companies if they have to get regulatory approvals for manufacturing and exporting medicines.

GBP Audit
It is well established in our experience of consulting with Pharma organizations that cGMP cannot be implemented without first establishing GBP. Establishing GBP requires extensive cultural transformations.
GBP Audit is a proprietary tool designed by UTSAV to enable Pharma organizations understand their readiness and maturity in implementing cGMP on enduring basis.
Read More

Distance Learning Program for GBP
With distance learning programme UTSAV aim to educate the Pharmaceutical executives at senior levels( with minimum ten years industry experience) on behavioural knowledge, skills and attitude necessary to implement GMP and QMS.
At the successful completion of programme, requiring written test and viva voce, the participants will be awarded certificate of completion of First Level GBP programme.

Case Studies
Read case studies of UTSAV clients who used our Good Behavioural Training Programs to drive their growth and build their brands.
A case study is a comprehensive study of a social unit of society, which may be a person, family group, institution, community or event. A case study focuses attention on a single unit thoroughly. The aim is that to find out the influencing factors of a social unit and the relationship between these factors and a social unit.

HR Communication & Newsletters
GBP based communication is a powerful tool to bring about change towards GBP. And it is an art few know how to implement in organization. We have developed a special expertise in the same and many clients have used it to their immense benefit. Please find below the actual samples of communication designed by us for the CEO of a Pharmaceutical orhganization.
Read More

Blog
Good Behavioural Practices can ensure competitive success for Indian Pharmaceutical industry. However, Indian Industrial culture is still to fully come out of ‘Chalta Hai’ mindset. Everyone knows that large numbers of Pharmaceutical plants need vast improvement in the work culture to compete in the world markets.
Read More

Pharma - Behavioural Issues
Pharmaceutical companies face complex issues that grow more challenging by the day
One of the most crucial questions facing the industry, though, is what leadership skills companies will need to navigate this complex and changing landscape—and how current pharmaceutical leaders stack up.
Read More

Video
"When you educate a man, you liberate a man." – Dr. Benjamin Carson.
Dr. Carson, a distinguished man of science and healing, the Director of the Pediatric Neurosurgery Division at the John Hopkins Hospital in Baltimore, talks about political correctness and how it can muzzle an entire nation. What is the point of an education if we do not voice our opinions on important issues?
Read More

 +91 9810009311
+91 9810009311 pradeepprakash2001@gmail.com
pradeepprakash2001@gmail.com





